NEW METHODS IN LEAD RECYCLING OF WASTE BATTERIES
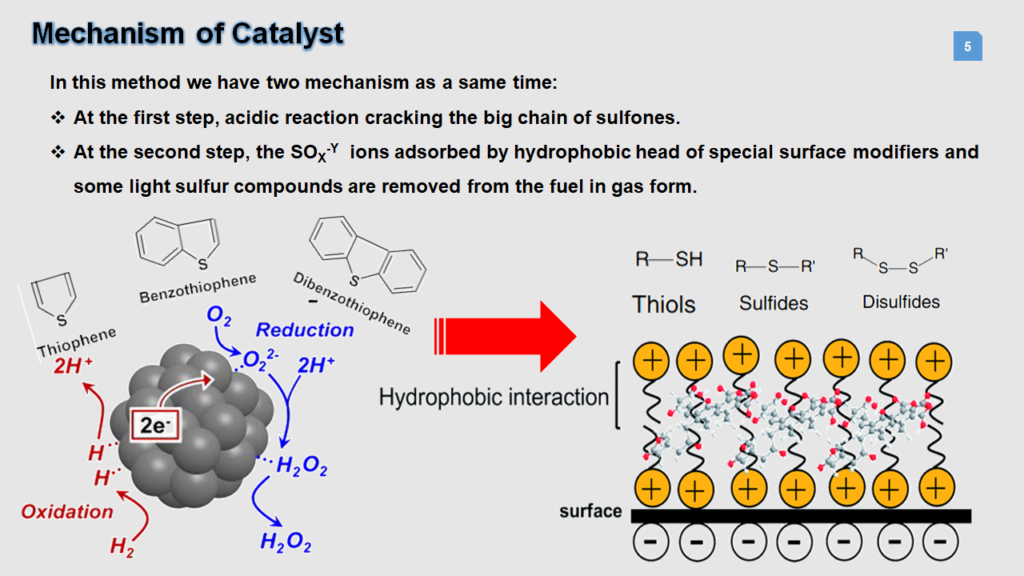
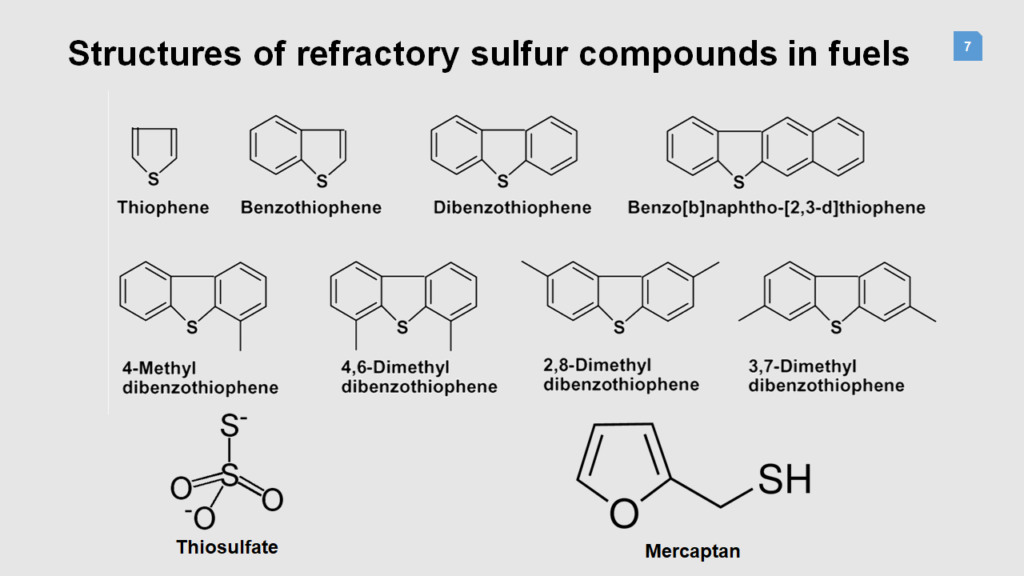
This product reveals the process for removing sulfur hydrocarbon compounds by using special functional porous ceramic-base nanoparticle adsorbents to remove unwanted sulfur hydrocarbon compounds which was performed at room temperature and ambient pressure. Various zeolite and silicate compounds with high porosity and adsorbing ability to remove sulfur components are produced using co-precipitation method and surface porous organic modifiers. Owing to the unique surface modification, the catalyst has the ability to break mercaptan and thiol bonds, convert them into oxides and adsorb the remained sulfur components from the surface. The presence of thiol and mercaptan functions in fuel makes ceramic base compounds suitable for adsorbing them on the surface depending on the fuel concentration and density. The emission of fuel sulfur components in the form of oxides and adsorbing the remained compounds by the catalyst with porous ceramic-base Nano adsorbent base reduce the unfavorable odor caused by sulfur compounds significantly.
Features of new ceramic adsorber
These new Super-adsorbents are able to adsorb a category of Sulfone petroleum compounds including naphtha, gasoline and petrol and etc. These adsorbents were synthesized at one stage and no sophisticated equipment is required. In this invention, the adsorption properties of the porous ceramic bases and the presence of fragile sulfur functions on the adsorbent surface have been simultaneously used to remove sulfur compounds from petroleum compounds at an ambient temperature. they can be easily added and separated from Hydrocarbon fuels by a simple method. The reduction level of sulfur in hydrocarbon fuels depends on the concentration of sulfur and the amount of the adsorbent, although chemical and rheological properties of any fuel are different.

Synthesis of new ceramic adsorber
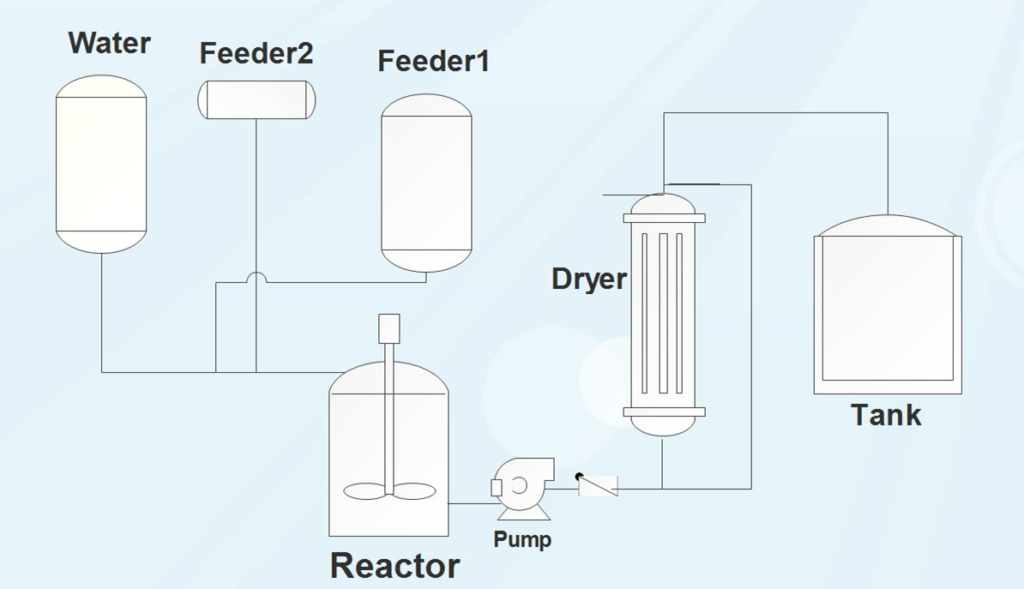
Synthesizing the adsorbent at an industrial scale requires a multi-feeder tank with the capability of liquid injection into the main tank. The tank is equipped with a stirrer Surface modifiers will be added to the system after mixing with water and condensation process will be carried out according to the charge level in the synthesis reservoir. The washing and drying procedure of the sample in the second tank is followed by the impregnation using the secondary superficial modifiers. In the next stage, the sample will be transferred to the dryer. The resulting vapor is collected by burial in water or re-distillation to prevent air pollution. The dryness of the applied adsorbent is very important
advantage:
- The cost of the production is not very high compared to the acid-washing methods and using oxidizing catalysts (ODS) and a molecular sieve adsorbent such as zeolites.
- The possibility of industrial production in mass quantities with exportation purpose.
- Unnecessary to spending too much time and applying a high temperature during the adsorbent production process.
- No requirement for utilizing complicated and expensive devices to manufacture, synthesis and using.
- No need to heat during the adsorption process, unlike oxidizing molecular sieve catalysts and the ability to adsorb at ambient pressure.
- No requirement for acid-washing and using oxidizing materials such as hydrogen peroxide and formic acid in the adsorption process
- The ability to be used as the adsorbent in a fixed context or mixed with the fuel.
- No requirement for re-purifying or changing the chemistry of the fuel
- According to international standards it is possible to produce petroleum compounds with a very low level of sulfur.
- No requirement for using sodium hydroxide and Caustic Soda in the adsorption process of sulfur from petroleum compounds.
- Restoring the adsorbent in order to reuse in the process.
- Ability to adsorb heavy metal elements from fuels such as lead and iron
Using of new ceramic adsorber
Method (1)
After the adsorbent addition to the fuel, a blender will be used for further mixing and shortening adsorption process duration. This blender can be used as either continuous cycle or rotating mixer. The adsorption process of sulfur from hydrocarbon fuels is followed by the separation of adsorbent from the fuel. For this purpose, two methods are carried out: in the first method, the adsorbent precipitates at the bottom of the tank due to its weight over time and the fuel will be placed on top. In this case, the pumps will easily separate the fuel. In second method, adsorbent is separated from the fuel by filtering and transferring to the recycling or burial stage. Then, after the completion of the adsorption process, the adsorbent will return to working cycle again by the recovering process along with some cheap aromatic solutions.
Method (2)
In the second method, the sulfur-containing fuel is pumped onto porous filters made from the adsorbent catalyst and passed through a pressure pump. In this case, adsorbent screens are fixed and fuel moves between these ceramic functionalized filters to separate sulfur. After a certain period, these filters are replaced and reused by the previous process.
Flow chart of desulfurization
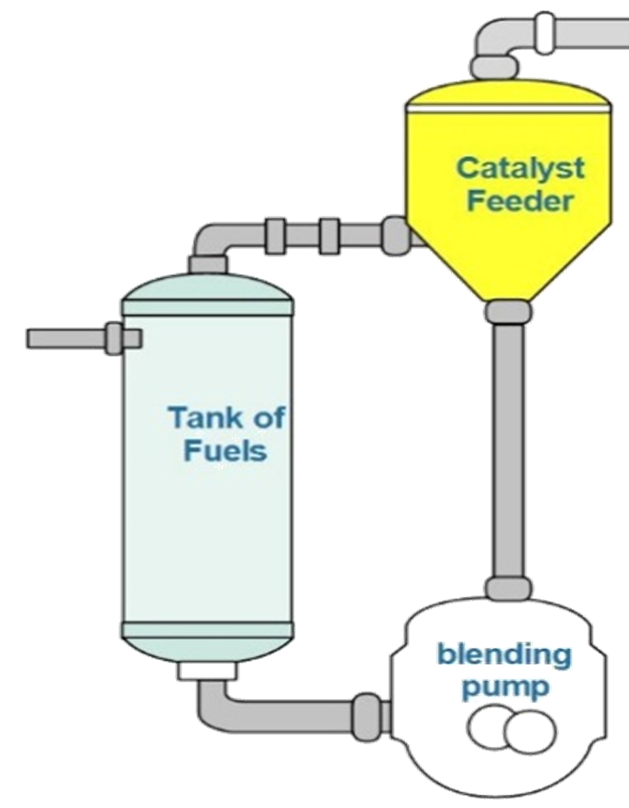
A simple schematic of how to mix the catalyst with the hydrocarbon fuels
